How Cells Find the Right Partners
Researchers at the University of Freiburg discover that the affinity between cells can control complex developmental processes
Fluorescence microscopy images of Drosophila egg chambers of different developmental stages. Eya in the epithelial cells is depicted in orange.Image: Vanessa Weichselberger/University of Freiburg
During the growth and development of living organisms, different types of cells must come into contact with each other in order to form tissues and organs together. A small team working with Prof. Dr. Anne Classen of the Excellence Cluster CIBSS – Centre for Integrative Biological Signalling Studies of the University of Freiburg has discovered that complex changes in form, or morphogenesis, during development are driven exclusively via the affinity of cells to each other. The researchers examined the egg chambers of fruit flies (Drosophila melanogaster) and combined genetic methods and mathematical modeling in their work. The study has been published in the scientific journal Nature Communications.
Complex organization processes in egg chamber
The lead author of the study and a member of Classen’s lab, Dr. Vanessa Weichselberger, summarized the team’s work: “We wanted to find out how different types of cells organize their morphogenesis with each other in order to form functional units.” She continues, “The egg chamber is a good example, because within it, different cell populations must self-organize into functional units.” The egg chamber is the structure in which an immature egg cell, or oocyte, matures until it is ready for fertilization. Drosophila’s egg chamber looks like a tiny football. Inside, the growing egg cell is located on one side, and on the other are 15 nurse cells that provide nutrients for the immature egg cell. In order to produce an egg, the egg cell must mature, while the nurse cells are ultimately removed.
Both processes – the maturation of the egg cell and the removal of the nurse cells, are dependent on an external layer of epithelial cells. For this purpose, the epithelial cells are divided into specialized groups, which – based on their function – must either make contact with the nurse cells or the egg cell. This partnering between the inner and outer cells is a complex process which takes place while simultaneously the size relationships within the egg chamber continually change. “Until now, the mechanisms that could robustly control such a dynamic process were unknown,” says Classen.
Eya controls the affinity of cells
The researchers observed that the epithelial cells specialized in removal of the nurse cells spread out and flatten over the nurse cells. This creates a particularly large contact area with the nurse cells underneath. Weichselberger explains, “That could be explained by heightened affinity between the two cell types. So we hypothesized that the matching of inner and outer cells took place through simple mechanical processes of attraction and repulsion.” A heightened affinity of one specialized group of epithelial cells to the nurse cells would lead to the rest of the epithelial cells being displaced from the nurse cells onto the egg cell. The researchers found that a protein, Eya, which can control the activity of genes, influences the contact behavior between epithelial cells and nurse cells. If the researchers increased the concentration of Eya in the epithelial cells, these increased their contact surface area with the nurse cells. If they removed Eya, the contact surface was minimized.
Cell affinity decisive for development
In order to test their hypothesis, the developmental biologists used mathematical models. To do this, they worked with Prof. Dr. Patrick Dondl of the Faculty of Mathematics and Physics of the University of Freiburg. Dondl created mathematical models that could simulate different degrees of mechanical affinity between the cells. “The mathematical models allowed us to show that a change in affinity dependent on Eya levels was sufficient to control the complex process of matching cell types,” explains Weichselberger. “That meant that we could use Eya as a set screw to genetically control partner location,” she says.
“Extremely flexible and robust”
By genetically changing the Eya concentrations in the epithelial cells and simulating these experiments on the computer, the researchers were able to test if the Eya-regulated affinity between the epithelial cells and nurse cells is responsible for self-organization. They observed that solely by manipulating Eya, they could deliberately control which epithelial cells spread out on the nurse cells and which epithelial cells came into contact with the egg cell. This showed that Eya – via affinity regulation – is the main regulator of self-organization between the epithelial cells and the inner cells – the nurse cells and the egg cell. The results surprised Classen, who led the study. She explains, “Specific affinity is actually sufficient as a mechanism for controlling such complex development processes. And in a way that is extremely flexible, robust, and independent from the volume of the egg chamber.”
Similar process in males
This mechanism is not limited solely to the egg chamber. The development of sperm cells in Drosophila males is also dependent on Eya. Here, too, the protein Eya controls the affinity between the developing, interior sperm cells and the exterior epithelial cells. It is unclear if these results can also be applied to other animals or humans. But comparable structures and developmental processes during oogenesis in other species make this seem possible.
About the Excellence Cluster CIBSS
The Excellence Cluster CIBSS – Centre for Integrative Biological Signalling Studies’ objective is to gain comprehensive understanding of biological signalling procedures regardless of scale, from interactions between individual molecules and cells to processes within organs and entire organisms. The researchers deploy the knowledge they obtain to develop strategies for targeted signal control. These technologies pave the way for new research findings and enable innovations in medicine and plant sciences.
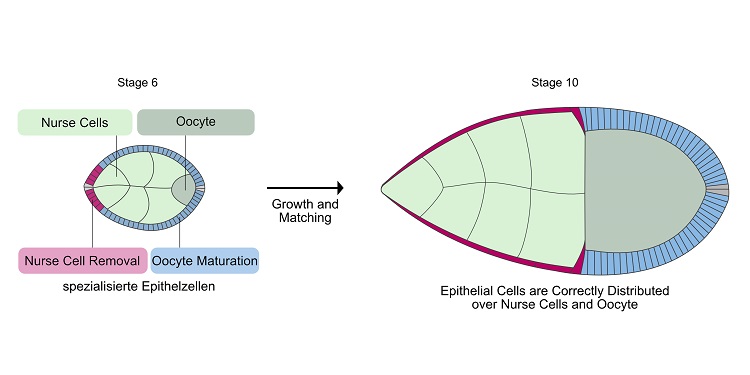 Illustration of Drosophila egg chambers in stage 6 and stage 10. Shown are nurse cells (light green), immature egg cell (dark green), epithelial cells specialized in nurse cell removal (red), and epithelial cells specialized in egg maturation (blue). At stage 10, the cells have found their correct partners. The red epithelial cells have spread over all nurse cells and the blue epithelial cells are in contact with the egg cell. Illustration: Vanessa Weichselberger/University of Freiburg
Illustration of Drosophila egg chambers in stage 6 and stage 10. Shown are nurse cells (light green), immature egg cell (dark green), epithelial cells specialized in nurse cell removal (red), and epithelial cells specialized in egg maturation (blue). At stage 10, the cells have found their correct partners. The red epithelial cells have spread over all nurse cells and the blue epithelial cells are in contact with the egg cell. Illustration: Vanessa Weichselberger/University of Freiburg
Factual overview:
Contact
Prof. Dr. Anne Classen
CIBSS – Centre for Integrative Biological Signalling Studies
University of Freiburg
+49 761 203 97190
anne.classen@zbsa.uni-freiburg.de
Michal Rössler
CIBSS – Centre for Integrative Biological Signalling Studies
University of Freiburg
+49 761 203 97120
michal.roessler@cibss.uni-freiburg.de


